Tuesday, November 25, 2008
Tuesday, November 18, 2008
A Case of Obscure Gastrointestinal Bleeding Secondary to a Small Bowel Gastr...
Sent to you by Hemi via Google Reader:
Magnetic resonance enterography may have a role in the evaluation of obscure gastrointestinal bleeding when upper and lower endoscopy is negative and capsule endoscopy is unrevealing.
The Medscape Journal of Medicine
Things you can do from here:
- Subscribe to Medscape Radiology Headlines using Google Reader
- Get started using Google Reader to easily keep up with all your favorite sites
Friday, November 14, 2008
Polyp Size and Advanced Histology in Patients Undergoing Colonoscopy
Tuesday, November 11, 2008
Small Colon Polyps: Surgical Removal Is Costly And Unnecessary
A decision analysis model was constructed to represent the clinical and economic consequences of performing three year colorectal cancer surveillance, immediate colonoscopy with polypectomy, or neither on patients who have 6-9 mm polyps found on CT colonography (CTC). The analysis model was accompanied by a hypothetical population of 100,000 60-year-old adults with 6- to 9-mm polyps detected at CTC screening. Results showed that, 'by excluding large polyps and masses, CTC screening can place a patient in a very low risk category making colonoscopy for small polyps probably not warranted,' said Perry J. Pickhardt, MD, lead author of the study. 'Approximately 10,000 colonoscopy referrals would be needed for each theoretical cancer death prevented at a cost of nearly $400,000 per life-year gained. We would also expect an additional 10 perforations and probably one death related to these extra colonoscopies. There may be no net gain in terms of lives - just extra costs,' said Dr. Pickhardt.
'The clinical management of small polyps detected at colorectal cancer screening has provoked controversy between radiologists and gastroenterologists. Patients should be allowed to have the choice between immediate colonoscopy and imaging surveillance for one or two isolated small polyps detected at colorectal cancer screening,' said Dr. Pickhardt.
CT colonography is now a recommended test for colorectal cancer screening by the American Cancer Society. 'If patients with small polyps are monitored, only five percent of adults undergoing CTC screening will need to undergo immediate invasive colonoscopy,' said Dr. Pickhardt.
This study appears in the November issue of the American Journal of Roentgenology.
Click here for abstract"
Virtual Colonoscopy: A Storm is Brewing
Abstract and Introduction
Abstract
The author performed the first virtual colonoscopy (VC) in 1993. In this article, he addresses the issues related to the turf battles between radiologists and gastroenterologists in the use of this technology. Reviewing common myths associated with VC, he warns that radiologists must retain expertise in this area.
Introduction
A storm is brewing around virtual colonoscopy (VC) and whether radiologists or gastroenterologists will ultimately control this technology. Imagine the following: in the near future, a patient who requires colorectal cancer (CRC) screening walks into a local gastroenterologist's office, obtains a VC examination, which is read by a nurse practitioner, and, following consultation with a gastroenterologist, undergoes immediate optical colonoscopy (OC) for evaluation of tiny polyps that either cannot be found or turn out to be residual feces. Meanwhile, a radiologist working with this practice interprets the CT data for extracolonic findings in exchange for a small percentage of the total professional fee. The patient's insurance (ie, Medicare) is billed for both the VC and OC, which taxpayers ultimately pay. If this sounds far-fetched, read on....
Virtual Colonoscopy Development
Colorectal cancer is the second leading cause of cancer death in the United States, but it is also one of the most preventable when screening is used to detect and treat early disease. The 5-year survival rate for early stage I CRC is 93%, but when it metastasizes to distant organs and becomes stage IV disease, the survival rate decreases to 8%.[1] Unfortunately, many adults over the age of 50 do not undergo screening, and, as a result, CRC is more often diagnosed in advanced stages.[2] Virtual colonoscopy offers the public a more appealing and less invasive alternative for screening.
I performed the first VC, also known as CT colonography (CTC), at the Wake Forest University Health Sciences Center in 1993. It has taken nearly 15 years for VC to mature and gain acceptance by policy makers. The basic technique consists of: 1) bowel cleansing and stool tagging, 2) gas insufflation of the colon, 3) CT scanning of the abdomen/pelvis, and 4) 2- and 3-dimensional image analysis of the data to identify polyps and masses (Figure 1). The first VC examination took 60 seconds to scan a patient using a single-slice helical CT scanner and nearly 8 hours to process the data for a fly-through, but today multidetector CT scanners acquire the data in a few seconds, and processing occurs in real time using inexpensive computers.
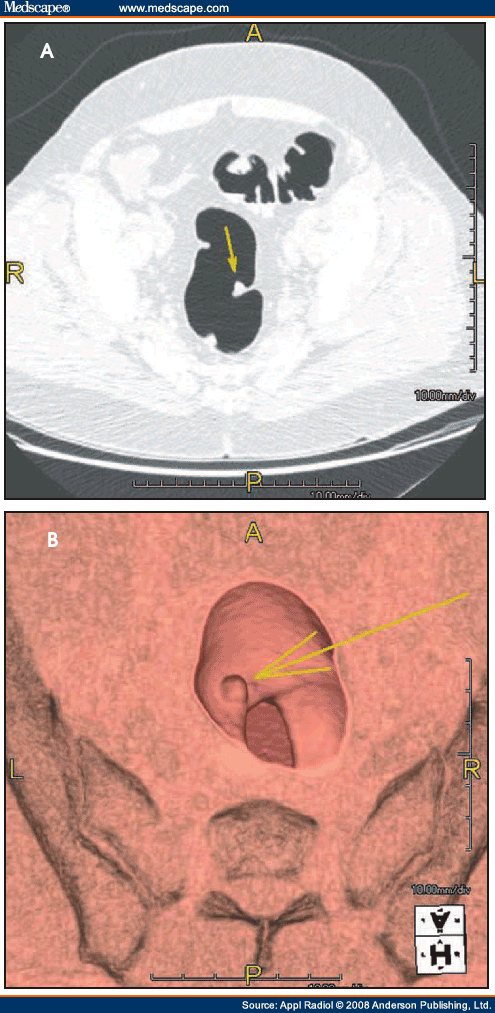
Figure 1.(A) This CT image shows a 10-mm polyp (arrow) on a fold in the sigmoid colon. (B) This 3-dimensional volume-rendered image shows the polyp (arrow) from a superior perspective.
Despite the technological advances that have occurred during the past decade (eg, CO2 insufflation, multidetector CT scanners, stool tagging, computer-assisted diagnosis), a strong lobbying effort on the part of gastroenterologists has delayed the availability of VC in the United States. Since Congress approved reimbursement for CRC screening in the 1997 Balanced Budget Act, the number of colonoscopies conducted annually in the United States has increased from 4 million in 2000 to >14 million in 2002.[3]
Handwriting on the Wall
Clinical trials that compared VC with OC have shown a dramatic improvement in VC accuracy in the last few years, culminating in 2 major trials that were announced in September 2007. The ACRIN National Colonography Trial enrolled over 2500 patients at 15 sites, and it reported that VC had a 90% sensitivity for the detection of polyps >10 mm.[4] Within a week, Kim[5] published a study comparing VC screening in 3120 patients with OC screening in 3163 patients. Remarkably, VC and OC found an equivalent number of advanced adenomas in each group; more surprisingly, a larger number of cancers were found in the VC group.[5] These 2 studies plus multiple prior published trials from the United States and abroad led the American Cancer Society, the American College of Radiology (ACR), and the United States Multi-Society Task Force to incorporate VC in its screening recommendations that were published in March 2008.[6]
As VC has gained acceptance, gastroenterologists now realize that VC will impact their practice. After years of bashing VC as not being good enough and requiring more clinical data, the Future Trends Committee of the American Gastroenterological Association (AGA) published a report in October 2006 stating that they see the handwriting on the wall.[7] This Committee proposed that gastroenterologists should position themselves to play a role in performing and interpreting VC, including advocating for CPT codes in the 91000 series that will allow gastroenterologists to be reimbursed for interpreting and providing VC services, as well as developing specialized training and training requirements for those interested in performing VC interpretation. In an effort to make good on its promise, the AGA published a set of guidelines in 2007 listing the minimum requirements that a gastroenterologist must satisfy in order to become certified to read VC examinations.[8]
Battle Lines are Drawn
Currently the Centers for Medicare and Medicaid Services (CMS) approve reimbursement for VC only when it follows a failed "diagnostic" colonoscopy, not a failed "screening" colonoscopy (Figure 2).[9] Following the inclusion of VC in the American Cancer Society's screening guidelines, CMS launched a National Coverage Analysis for Screening Computed Tomography Colonography for Colorectal Cancer (CAG-00396N) in May 2008. This seeks to expand reimbursement for screening indications. The final report of this analysis is due in February 2009.[10] Expanded reimbursement could have a huge impact on increasing screening and reducing CRC deaths, but it could also have substantial economic consequences for CMS and taxpayers. A public comment period held May-June 2008 drew responses from many individuals and organizations, including the ACR and the AGA. Of course, the ACR is in favor of expanded reimbursement, but the AGA stated that it would support VC only if certain conditions were met,[11] including:
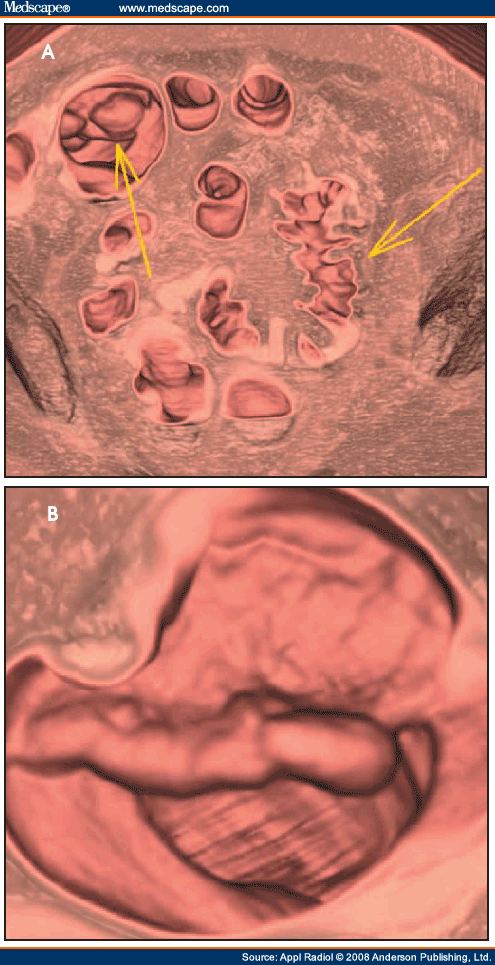
Figure 2.(A) Virtual colonoscopy (VC) shows a diverticular stricture (right arrow) that prevented colonoscopy from successfully evaluating the entire colon. However, VC was able to identify an ascending colon cancer (left arrow) that was the cause of the patient's occult gastrointestinal bleeding. (B) A close-up of the ascending colon cancer from a superior view.
1. Reporting of ALL polyps (which is contradictory to the ACR Practice Guideline for the Performance of CTC in Adults that states reporting of polyps <5 mm is not recommended because of the low incidence of those lesions having malignant potential);[12]
2. Allowing patients in consultation with their physician to determine whether or not to remove those polyps; and
3. Enacting a coverage policy that would encourage rapid follow-up procedures (ie, colonoscopy) and that correspondingly would not create a disincentive for physicians (ie, gastroenterologists) who refer those procedures.
Reading between the lines, if such conditions are approved by CMS, then the gastroenterologists will have an unrestrained ability to perform colonoscopy on any little lump or bump that they might discover if they or their clinical assistant should be allowed to read VC exams. It is also the position of many prominent gastroenterologists to create a split-fee arrangement with radiologists so that radiologists will be relegated to reading only the extracolonic portions of a CT scan for a small portion of the professional fee, and, if radiologists refuse to participate, then they will outsource radiology services, even to foreign providers![13]
Dispelling Popular Myths
Gastroenterologists frequently try to discredit VC with the following myths:
1. Colonoscopy is the "gold standard." There are no published studies to validate this claim. In fact, studies comparing back-to-back colonoscopies on the same patients have reported OC miss rates of 22% for polyps, even in the hands of expert endoscopists.[14] Studies such as Pickhardt's[15] landmark VC study have shown VC to outperform OC. Finally, the accuracy of screening colonoscopy has been shown to be dependent on how much time a gastroenterologist spends performing the examination.[16]
2. If VC finds a polyp, then colonoscopy is needed for polyp removal, so why not undergo colonoscopy in the first place? The vast majority of polyps are benign, hyperplastic polyps, and <5% of the asymptomatic screening population has a significant adenomatous polyp.[5] Hence, if OC is the primary screening method, then >95% of the asymptomatic population would under go OC unnecessarily with its inherent risks of bowel perforation and anesthesia.
3. The radiation dose associated with VC is prohibitive. Radiation dose is a valid concern, but researchers are striving to mitigate this risk by using low-dose techniques, even as low as 10 mAs (compared with a conventional CT scan that might use a dose of 200 mAs).[17] Hence, the radiation risk from VC with low-dose techniques can be on the order of 1 to 2 mSv, which is far below the range that has been associated with potential cancer and multidetector CT use.[18] Alternatively, VC can be performed using MRI, but the availability of MRI scanners is a temporary hurdle, at least for today.
Actions to Take
Radiologists are already overworked due to the exponential increase in imaging studies during the past decade, and as a result, we have become complacent about the ownership of new technologies. In the meantime, gastroenterologists are purchasing CT scanners and attending training programs to get ready for CMS approval of reimbursement for VC screening.[19] However, if radiologists act quickly and take certain steps to position ourselves to maintain control of VC, we will not risk losing this technology, as we have done with cardiac imaging. Some initiatives include:
1. Taking a stronger, vocal interest in VC. Radiologists are better trained to read an entire CT examination, especially when disease crosses organ boundaries to involve both the colon and adjacent anatomy. We need to establish ourselves as the imaging experts in order to counter claims that endoscopists and nurse practitioners are as good as radiologists in reading VC exams.[20]
2. Beginning a dialogue with community gastroenterologists and primary care physicians. Radiology practices need to be willing to provide same-day, on-demand VC services for failed "diagnostic" colonoscopy examinations in advance of the anticipated reimbursement for screening VC.
3. Developing practice guidelines for appropriately working-up extracolonic findings. Perhaps offering immediate but limited ultrasound evaluation to resolve indeterminate liver and renal lesions will help to mitigate the gastroenterologists' cry that they should be the ones performing VC in their offices.
4. Providing consistent, high-quality reports of VC findings that can be rapidly delivered to the patient and referring clinician. Utilization of the CT Colonography Reporting and Data System (C-RADS) and participation in the ACR's CTC Registry will help to strengthen our position in the field.[21,22]
5. Challenging any proposals by gastroenterologists to split the professional fee for reading colonic and extracolonic portions of a VC CT scan, including legislative lobbying if necessary. There are many problems with fee-splitting arrangements, not the least of which is malpractice liability—radiologists will certainly be held liable when gastroenterologists fail to make a correct diagnosis if they should be allowed to interpret only the intraluminal portion of a VC scan.
All is Not Lost, at Least Not Yet
Much of the rhetoric coming from the gastroenterology community is coming from a few but very vocal and rabid gastroenterologists. In fact, a survey of 2400 AGA members regarding their interest in VC resulted in only 588 responses, of which one third said that they would want to perform VC, another third said that they would not perform it but would support their colleagues, and the final third said that gastroenterologists should not perform VC.[23] In reality, radiologists and gastroenterologists will need to work together along with surgeons and oncologists to provide comprehensive CRC screening and treatment services. If CRC screening really takes off, then there will not be enough gastroenterologists available in this country to perform the necessary therapeutic colonoscopies that will be generated. Although radiologists specializing in VC may eventually become employees of large, multispecialty clinics specializing in colorectal disease, it is paramount that the role and expertise of the radiologist be maintained.
--
www.hwsfinance.blogspot.com/
NAI
In a child younger than two years of age, any suspicion of child abuse requires a skeletal survey and a head CT. In a child older than 2, symptom-specific imaging is recommended; brain MR or head CT can also be considered to evaluate for remote injury. Bone scan is sometimes useful, usually in the setting of equivocal radiographic findings or delayed workup. Bone scan is more sensitive for rib fractures, but less sensitive for skull and metaphyseal fractures.
Certain clinical findings should raise the suspicion of child abuse. These include multiple fractures, especially of different ages, bruising greater than expected for the patient's age, burns (often in the shape of common objects), bite marks, retinal hemorrhage, injury inconsistent with history, and delay in seeking care.
Fractures have varying levels of specificity for child abuse. The most common fracture in abuse is a long bone shaft fracture, which is not specific for abuse, except in infants younger than 9 months. Metaphyseal "bucket handle" or "corner" fractures have a high specificity for abuse. These occur most frequently at the knee (distal femur or proximal tibia and fibula), ankle (distal tibia) and shoulder (proximal humerus). Posterior rib fractures also have a high specificity. Rib fractures occur in 5-27% of abuse victims; 90% of all rib fractures occur in patients younger than 2 years. Other high specificity fractures include scapular and sternal fractures. Skull fractures are not well-correlated with abuse, as they are common in accidental trauma. Skull fracture findings concerning for abuse include multiple fractures and stellate fractures.
Brain injury is the leading cause of morbidity and mortality in nonaccidental trauma. Injury patterns include subarachnoid, subdural, and intraparenchymal hemorrhage. Diffuse cerebral edema and diffuse axonal injury can also be seen. An interhemispheric subdural hematoma is very concerning for abuse.
Abdominal injury related to child abuse primarily occurs in children older than 2. It is usually the result of blunt force trauma. It accounts for approximately 20% of the fatalities related to abuse. The most common finding is injury to the duodenum and proximal jejunum; mural hematoma may be seen. Another injury worthy of mention is pancreatitis; traumatic pancreatitis is the second most common etiology in kids. Other abdominal findings include solid organ lacerations and contusions, adrenal hematoma, and bladder rupture.
--
www.hwsfinance.blogspot.com/
Cerebral venous thrombosis
Discussion:
 Figure 2. (Click to enlarge) |  Figure 3. (Click to enlarge) |
Please note: in Figures 2 and 3, Ss S= Superior sagittal sinus, Co S= Confluence of sinus, Si S= Sigmoid sinus, and T S= Transverse sinus.
This patient had an MRV that showed a superior sagittal sinus thrombosis. Lack of signal from the distal portion of the superior sagittal sinus was consistent with cerebral venous thrombosis, as was the clinical presentation of this patient. A workup for a hypercoagulable state was negative. Cerebral venous thrombosis (CVT) should be considered in any patient presenting with severe headache and vomiting when no other cause is identified, especially when papilledema is identified on ophthalmoscopy. Risk factors for CVT include trauma (head injury), iatrogenic causes (craniotomy, jugular catheterization, lumbar puncture), systemic conditions (pregnancy, puerperium, dehydration), infections (otitis, sinusitis), inherited or acquired hypercoagulable states (protein C or S deficiency, antiphospholipid antibody syndrome, antithrombin III deficiency, factor V Leiden), collagen vascular diseases (systemic lupus erythematosus, Wegener granulomatosis), hematologic conditions (polycythemia, paroxysmal nocturnal hemoglobinuria, sickle cell disease), and medications (oral contraceptives, hormone therapy, steroids); however, no risk factors are identified in 14% of patients diagnosed with CVT.[6,7,8]
The typical pathophysiology in CVT is partial or complete occlusion of a cerebral venous sinus, which causes increased intracranial pressure and papilledema. The most frequently involved venous sinuses are the superior sagittal sinus or either of the paired transverse sinuses. If a thrombus extends into the cortical veins, dilatation of veins and capillary beds can occur, which then can lead to venous infarction, cerebral edema, and intraparenchymal hematoma formation. Seizures or cerebral herniation are potentially fatal complications.[7]
The clinical presentation of CVT is usually that of a severe headache that is aggravated by head movements, sneezing or coughing. The headache is typically subacute and gradual in onset, although thunderclap headache occurs in 2-10% of cases (as in this patient). Nausea and vomiting are often present, and severe cases are marked by a decreasing level of consciousness that may result from either a postictal state from seizures or from cerebral swelling with or without herniation. Focal neurologic findings, including hemiparesis or isolated unilateral lower extremity weakness, can be seen when a thrombus extends into the cortical veins. This is especially common in CVT of the superior sagittal sinus. If the cavernous sinus is involved (which is almost exclusively associated with infection of the paranasal sinuses), proptosis and chemosis with ipsilateral periorbital edema, retinal hemorrhages, papilledema, extraocular movement abnormalities, and sensory loss in the V1 or V2 distribution of the trigeminal nerve may be seen. Isolated or multiple cranial nerve palsies (III, VII, VIII) have been reported in patients with unilateral occlusion of the transverse or sigmoid sinuses. This unusual presentation has been explained by venous congestion of the ventral pontine and lateral medullary veins. If the thrombus extends into the jugular bulb, a jugular foramen syndrome, with involvement of cranial nerves IX, X and XI, may be seen. The physical examination is normal in 15-30% of patients, although careful examination may reveal loss of normal spontaneous retinal venous pulsations. It has been has reported that headache (89%), paresis (37%), generalized (30%) or focal (20%) seizures, papilledema (28%), and mental status changes (22%) are the most frequent presenting symptoms and signs associated with the diagnosis of CVT.[1,6,7,8]
The differential diagnosis of CVT includes subarachnoid hemorrhage, intracranial hemorrhage, ischemic stroke, and bacterial meningitis. In patients presenting with the more typical subacute onset, pseudotumor cerebri, cavernous sinus syndromes, intracranial abscess, subdural empyema, and brain tumor should be considered.[7,8]
The definitive diagnosis of CVT is demonstration of the thrombus by neuroimaging. Noncontrast-enhanced CT scans of the head are normal in 25% of patients with a normal examination and 10% of patients with focal neurologic findings (including papilledema). The delta sign is a dense triangle in the superior sagittal sinus caused by the thrombus, and it may be seen on CT scans. MRV is considered the radiographic investigation of choice and will demonstrate areas of lack of signal where the thrombus involves the venous sinuses. Cerebral angiography may be required if MRV is non-diagnostic and suspicion remains high. CT venography is a method that is increasingly being used to diagnose CVT, and it has been shown to be very sensitive and specific. The empty delta sign is a triangular filling defect in the superior sagittal sinus that complements the delta sign on noncontrast-enhanced CT scans. Lumbar punctures are sometimes performed in patients with no evidence of mass effect on CT scans. About 50% of patients will have abnormal cerebrospinal fluid (CSF) findings, including mild lymphocytic pleocytosis, elevated protein, the presence of red blood cells, and, more commonly, an elevated CSF opening pressure (which must be measured with the patient as relaxed as possible in the lateral decubitus position).[3,4,6,7,8]
The mainstay of treatment for CVT, even in patients with CT evidence of cerebral ischemia or hemorrhage, is anticoagulation. In 2 retrospective studies of patients with CVT and moderate-sized hematomas, anticoagulation was not associated with increased hemorrhage volume, neurologic deterioration, or a worse outcome. Heparin should be started initially, with a goal of maintaining an activated partial thromboplastin time that is twice the control value. Heparin therapy is gradually transitioned to warfarin, which is then continued for 4-6 months (longer in patients with an identified predisposition to clotting). Endovascular thrombolytic therapy with urokinase or tissue plasminogen activator (tPA) may be effective in patients who deteriorate despite adequate anticoagulation with heparin, but this therapy is limited to specialized centers. Surgical intervention, in the form of a thrombectomy using a microsnare or rheolytic thrombectomy catheter with local thrombolytic therapy, has been used with variable success in the setting of severe neurologic deterioration. Adjunctive therapy for seizures, cerebral edema or co-occurring infections may also be required on a case-by-case basis. Headaches related to increased intracranial pressure may respond to elevation of the head of the bed or to acetazolamide, which decreases CSF production (thereby lowering intracranial pressure). When complicated by visual deterioration unresponsive to these measures, lumboperitoneal shunting or optic nerve sheath fenestration should be considered.[4,6,7]
The complications of CVT may include coma (resulting from status epilepticus, critically elevated intracranial pressure, or impending cerebral herniation) and pulmonary embolism. In patients who are comatose as a result of raised intracranial pressure, prompt intervention is critical. Initial measures include maintaining the head of the patient at 30-40° elevation, keeping the neck in a neutral position to avoid kinking of the jugular veins, and the use of mannitol or hyperventilation. Additional therapy, such as ventriculostomy and blood pressure titration with vasoactive agents, should be guided by direct intracranial pressure monitoring. Pulmonary embolisms occur in up to 11% of cases and may originate from the thrombosed jugular veins or from other sites (such as the legs). This complication carries a high mortality rate.
The prognosis is good for patients with CVT that is recognized early. Full recovery is expected in about 70% of cases. Of the remaining 30%, about one third die and two thirds are left with persistent mild-to-moderate neurologic deficits. Coma, seizures, or underlying disease have no significant effect on the short-term outcome and should not preclude any type of therapeutic intervention. The long-term recurrence rate of CVT is approximately 20%. Oral contraceptives are a recognized risk factor for CVT; this is supported by the increasing rate of CVT in women of childbearing age since introduction of oral contraceptives, with stable rates seen in men of similar age.[7]
In the case presented above, the patient was treated with analgesics and low-molecular-weight heparin, followed by warfarin. She discontinued use of her oral contraceptive pills. The patient recovered completely, with no residual deficits, and she continues to do well on long-term follow-up.
--
www.hwsfinance.blogspot.com/
Monday, November 10, 2008
MRI of Temporomandibular Joint Disorders
Sent to you by Hemi via Google Reader:
Magnetic resonance imaging (MRI) plays an important role in the evaluation of temporomandibular disorders (TMD). What are the MRI appearances of the degenerated temporomandibular joint?
Applied Radiology
Things you can do from here:
- Subscribe to Medscape Radiology Headlines using Google Reader
- Get started using Google Reader to easily keep up with all your favorite sites
Proximal Vein Compressibility on Ultrasound Sufficient for Diagnosing DVT
Sent to you by Hemi via Google Reader:
For symptomatic outpatients with suspected deep vein thrombosis (DVT) of the lower extremities, results of a prospective clinical trial suggest that "2-point ultrasonography," or "compression ultrasonography," evaluating compressibility of the proximal veins is as accurate as whole-leg Doppler ultrasonography.
Reuters Health Information
Things you can do from here:
- Subscribe to Medscape Radiology Headlines using Google Reader
- Get started using Google Reader to easily keep up with all your favorite sites
Radiological Case: Pigmented Villonodular Synovitis of the Elbow
Sent to you by Hemi via Google Reader:
Increasing elbow pain and swelling were present in a 73-year-old woman who was diagnosed with pigmented villonodular synovitis of the elbow. What was the preferred diagnostic imaging modality?
Things you can do from here:
- Subscribe to Medscape Radiology Headlines using Google Reader
- Get started using Google Reader to easily keep up with all your favorite sites
Radiological Case: Budd-Chiari Syndrome
Sent to you by Hemi via Google Reader:
Right upper quadrant pain was present in a 77-year-old woman who was diagnosed with Budd-Chiari syndrome. Which imaging modalities were used to evaluate her pain and diagnose her condition?
Applied Radiology
Things you can do from here:
- Subscribe to Medscape Radiology Headlines using Google Reader
- Get started using Google Reader to easily keep up with all your favorite sites
CT Important in Emergency Diagnosis of Appendicitis
Sent to you by Hemi via Google Reader:
CT of the appendix has a significant impact on the management of emergency department patients who are suspected of having appendicitis, researchers report in the October issue of the American Journal of Roentgenology.
Reuters Health Information
Things you can do from here:
- Subscribe to Medscape Radiology Headlines using Google Reader
- Get started using Google Reader to easily keep up with all your favorite sites
Online Radiological Case: Idiopathic Epidural Lipomatosis
Sent to you by Hemi via Google Reader:
A history of compressive myelopathy is present in a 35-year-old man diagnosed with idiopathic epidural lipomatosis. What's the preferred imaging modality for diagnosis and follow-up of this condition?
Applied Radiology
Things you can do from here:
- Subscribe to Medscape Radiology Headlines using Google Reader
- Get started using Google Reader to easily keep up with all your favorite sites
Facet Joint Osteoarthritis and Low Back Pain in the Community-Based Population
Sent to you by Hemi via Google Reader:
Is there an association between lumbar spine facet joint osteoarthritis and low back pain? See the results from this community-based Framingham Heart Study.
Spine
Things you can do from here:
- Subscribe to Medscape Radiology Headlines using Google Reader
- Get started using Google Reader to easily keep up with all your favorite sites
Thursday, November 6, 2008
.
Lumbar Degenerative Disk Disease1
Michael T. Modic, MD and Jeffrey S. Ross, MD1 From the Division of Radiology, Cleveland Clinic Foundation, 9500 Euclid Ave, Cleveland, OH 44195. Received October 31, 2005; revision requested December 1; revision received February 15, 2006; accepted March 10; final version accepted June 8; final review and update by M.T.M. March 28. Address correspondence to M.T.M. (e-mail: modicm1@ccf.org).
| ABSTRACT |
|---|
| |
|---|
The sequelae of disk degeneration are among the leading causes of functional incapacity in both sexes and are a common source of chronic disability in the working years. Disk degeneration involves structural disruption and cell-mediated changes in composition. Mechanical, traumatic, nutritional, and genetic factors all may play a role in the cascade of disk degeneration, albeit to variable degree in different individuals. The presence of degenerative change is by no means an indicator of symptoms, and there is a very high prevalence in asymptomatic individuals. The etiology of pain as the symptom of degenerative disease is complex and appears to be a combination of mechanical deformation and the presence of inflammatory mediators. The role of imaging is to provide accurate morphologic information and influence therapeutic decision making. A necessary component, which connects these two purposes, is accurate natural history data. Understanding the relationship of etiologic factors, the morphologic alterations, which can be characterized with imaging, and the mechanisms of pain production and their interactions in the production of symptoms will require more accurate and reproducible stratification of patient cohorts.
© RSNA, 2007
| INTRODUCTION |
|---|
| |
|---|
It has been stated that the term degeneration, as it is commonly applied to the intervertebral disk, covers such a wide variety of clinical, radiologic, and pathologic manifestations as to be really "only a symbol of our ignorance" (1). Despite this admonition, or perhaps because of it, we will attempt in this review to summarize current thoughts about the etiology, manifestations, and symptom production in lumbar degenerative disk disease and the role imaging currently plays in its identification and management. Given the gaps in our knowledge and the complexity of the subject, which will become readily apparent, we have chosen to exclude detailed treatment options from the discussion.
The sequelae of disk degeneration remain among the leading causes of functional incapacity in both sexes and are a common source of chronic disability in the working years. In accordance with its incidence, morbidity, and socioeconomic impact, degenerative disk disease has given and continues to give rise to extensive research efforts into its epidemiology, anatomy, biomechanics, biochemistry, and neuromechanisms (2).
| ETIOLOGY |
|---|
| |
|---|
Traditionally, disk degeneration has been linked to mechanical loading. The importance of mechanical factors has been emphasized by experiments on cadaver spines with both a severe single event and relentless loading (3–7). Failure of disks is more common in areas where there are the heaviest mechanical stresses, such as the lower lumbar region. It has been suggested that mechanical factors produce endplate damage, the antecedent to disk degeneration (8).
The disk is metabolically active, and the metabolism is dependent on diffusion of fluid either from the marrow of the vertebral bodies across the subchondral bone and cartilaginous endplate or through the annulus fibrosus from the surrounding blood vessels. Morphologic changes in the vertebral bone and cartilaginous endplate, which occur with advancing age or degeneration, can interfere with normal disk nutrition and further the degenerative process. This disruption of the normal endplate results in deformation when under loading. This allows nuclear material to pass through the endplate, reducing intradiscal pressure with subsequent bulging and loss of height and adding more stress to the surrounding annulus. Compressive damage to the vertebral body endplate alters the distribution of stresses in the adjacent disk. Continual cyclic loading makes these changes worse. Diminished blood flow in the endplate initiates tissue breakdown first in the endplate and then in the nucleus. These altered stress distributions adversely affect disk cell metabolism. These changes then alter the integrity of the proteoglycans and water concentration, reducing the number of viable cells with subsequent alteration in the movement of solutes into and out of the disk (9).
The importance of normal blood flow to the homeostatic nutritional process in the intervertebral disk complex has been suggested to explain the association of atherosclerosis and aortic calcification with increased disk degeneration and subjective low back pain (10). As degeneration progresses, structures of the disk become more disarranged and greater stresses are placed on the annulus and facet joints. As increased forces are transmitted to the annulus, there may be fragmentation and fissuring. Disk degeneration involves structural disruption and cell-mediated changes in composition, but which occurs first is not clear. Biochemical factors can increase susceptibility to mechanical disruption, and this could adversely influence disk cell metabolism. Regardless of the initiating mechanism, these mechanisms would be interactive and additive, the end result being an altered functional ability of the disk to resist applied forces.
In addition to mechanical and nutritional causes, a genetic predisposition has been suggested by animal models that consistently develop degenerative disk disease at an early age, as well as by reports of familial osteoarthritis and lumbar canal stenosis in humans (11). In a study (12) of 115 male identical twin pairs, the effects of lifetime exposure to commonly suspected risk factors on disk degeneration, including job type, lifting, twisting, sitting, driving, exercise, trauma, and cigarette smoking, were investigated. The particular environmental factors studied, which have been among those most widely suspected of accelerating disk degeneration, had only modest effects. These small effects would help to explain the mixed results of previous studies. Considering the very minor effects the particular environmental factors studied had in determining disk degeneration, a strong genetic influence was suggested (12). While failing to find a strong association or clear-cut etiologic influence, the authors concluded that disk degeneration may be explained primarily by genetic influences and by unidentified factors, which may include complex unpredictable interactions.
In a cohort study (13) based on a Danish twin registry, substantial genetic influence on the susceptibility to degenerative disk disease and low back pain was shown. Shared environment was important until the age of 15 years, but as the twins grew older, the effect of a nonshared environment increased and nonadditive genetic effects became more evident. These findings suggest increasing genetic interaction (13).
Abnormalities of collagen are most often cited to support genetic influence in degenerative disk disease. Type II collagen is the most abundant collagen of cartilaginous tissues and is often referred to as the major collagen, forming heterotypic fibrils with the less abundant minor collagen types IX and XI. These fibrils provide the strength necessary to resist tensile forces. Disease that causes mutations in types II and XI collagen has been demonstrated in a number of chondrodystrophies. Multiple epiphyseal dysplasia is associated with a disease causing mutation in collagen IX, an important structural component of the annulus fibrosus, nucleus pulposus, and hyaline cartilage of the endplates. In a study looking for additional disease-producing mutations, researchers closely examined the genes coding the three chains of type IX collagen. While no changes typical of disease producing mutations were identified, two amino acid substitutions were identified that are substantially more prevalent in patients with lumbar degenerative disk disease than in normal controls. Both of these involve a tryptophan substitution (Trp2 and Trp3). In the case of the Trp3 allele, it is a genetic factor associated with a threefold increase in the risk of symptomatic degenerative disk disease (14–16). While these Trp alleles together occurred in 16% of the Finnish patients with lumbar disk disease, it has been pointed out that other loci are almost certainly involved. A prime candidate is one that encodes aggrecan, an abundant proteoglycan, in cartilage whose extensive hydration contributes to resistance to tissue deformation. Knock-out mice for aggrecan have a high prevalence of disk herniation and degeneration (15,17).
Several additional studies (18–21) suggest that not just the process of degenerative disk disease but perhaps even its sequelae, including disk herniation, low back pain, and radiculopathy, are strongly influence by genetic factors. Studies suggest that low back pain is also associated with polymorphism in the interleukin (IL) 1 locus (22). This is of interest in that cytokines such as tumor necrosis factor  (TNF-
(TNF- ), IL-1, and IL-6 are important inflammatory mediators, as will be discussed latter.
), IL-1, and IL-6 are important inflammatory mediators, as will be discussed latter.
Clearly there are many interactive factors at play. Mechanical, traumatic, nutritional, and genetic factors all may play a role in the cascade of disk degeneration, albeit to variable degrees in different individuals. Whatever the etiology, by the age of 50 years, 85%–95% of adults show evidence of degenerative disk disease at autopsy (23).
| MORPHOLOGIC ALTERATIONS AND IMAGING |
|---|
| |
|---|
Terminology
No less a problem than understanding etiology is agreeing on terminology that is reliable and reproducible to describe the morphologic alterations produced by the degenerative process (24–27). For the purposes of this review, we have used the terminology described by Milette (28). The term degeneration includes any or all of the following: real or apparent desiccation, fibrosis, narrowing of the disk space, diffuse bulging of the annulus beyond the disk space, extensive fissuring (ie, numerous annular tears) and mucinous degeneration of the annulus, defects and sclerosis of the endplates, and osteophytes at the vertebral apophyses. At magnetic resonance (MR) imaging, these changes are manifested by disk space narrowing, T2-weighted signal intensity loss from the intervertebral disk, presence of fissures, fluid, vacuum changes and calcification within the intervertebral disk, ligamentous signal changes, marrow signal changes, osteophytosis, disk herniation, malalignment, and stenosis. While there is confusion in the differentiation of changes of the pathologic degenerative process in the disk from those of normal aging, we will use the term degenerative to include all such changes (29–31).
Conventional theory would imply that degeneration and aging are very similar processes, albeit occurring at different rates (32). Resnick and Niwayama (32) emphasized the differentiating features of two degenerative processes involving the intervertebral disk, which had been previously described by Schmorl and Junghanns (33). These include "spondylosis deformans," which affects essentially the annulus fibrosus and adjacent apophyses, and "intervertebral osteochondrosis," which affects mainly the nucleus pulposus and the vertebral body endplates but also includes extensive fissuring (numerous tears) of the annulus fibrosus. Scientific studies suggest that spondylosis deformans is the consequence of normal aging, whereas intervertebral osteochondrosis, sometimes also called deteriorated disk, results from a clearly pathologic, through not necessarily symptomatic, process (33–38). Anterior and lateral marginal vertebral body osteophytes have been found in 100% of skeletons of individuals over 40 years of age, and therefore are consequences of normal aging, whereas posterior osteophytes have been found in only a minority of skeletons of individuals over 80 years, and therefore are not inevitable consequences of aging (34). Endplate erosions with osteosclerosis and chronic reactive bone marrow changes also appear to be pathologic.
Anatomic Considerations
The intervertebral joint is a three-joint complex consisting of the endplate-disk-endplate joint of the anterior column and the two facet joints of the posterior column supported by ligaments and muscle groups. Understanding the interrelationship of these elements has become more critical as surgical intervention, much like joint surgery in the past, is transitioning from fusion to joint replacement.
The intervertebral disk and the diarthrodial joints (zygoapophyseal joint or facet joints) interactively degenerate, causing altered stresses on the integrity and mechanical properties of the spinal ligaments, which results in degeneration of the spinal unit as a whole (39,40). The manner of degeneration of the various components of the spine is mediated and manifested by the specific structure involved. The cartilaginous, synovial, and fibrous structures each degenerate in a specific manner, which is associated with characteristic imaging and pathologic aberrations.
Degenerative Disk Changes
The major cartilaginous joint (amphiarthrosis) of the vertebral column is the intervertebral disk. Each disk consists of an inner portion, the nucleus pulposus, surrounded by a peripheral portion, the annulus fibrosus. The nucleus pulposus is eccentrically located and more closely related to the posterior surface of the intervertebral disk. With degeneration and aging, type II collagen increases outwardly in the annulus and there is a greater water loss from the nucleus pulposus than from the annulus. This results in a loss of the hydrostatic properties of the disk, with an overall reduction of hydration in both areas to about 70%. In addition to water and collagen, the other important biochemical constituents of the intervertebral disk are the proteoglycans. The individual chemical structures of the proteoglycans are not changed with degeneration, but their relative composition is. The ratio of keratin sulfate to chondroitin sulfate increases, and there is a diminished association with collagen that may reduce the tensile strength of the disk. The decrease in water-binding capacity of the nucleus pulposus is thought to be related to the decreased molecular weight of its nuclear proteoglycan complexes (aggregates). The disk becomes progressively more fibrous and disorganized, with the end stage represented by amorphous fibrocartilage and no clear distinction between nucleus and annulus (41–43). On T2-weighted images, the central disk signal intensity is usually markedly decreased and at distinct variance to that seen in unaffected disks of the same individual. Work with T2-weighed spin-echo (SE) sequences (44) suggests that MR is capable of depicting changes in the nucleus pulposus and annulus fibrosus relative to degeneration and aging based on a loss of signal intensity.
In work with cadaver spines of various ages, absolute T2 measurements correlated more closely with glycoaminoglycan concentration than absolute water content. Thus, the signal intensity may not be related to the total amount of water but rather the state of water. At present, the role that specific biochemical changes (proteoglycan ratios, aggregation of complexes) play in the altered signal intensity is not well understood. Given that the T2 signal intensity in the disk appears to track the concentration and regions of high glycoaminoglycan percentages more than absolute water content, it seems likely that the health and status of the proteoglycans are major determinates of signal intensity (45).
It has been proposed that annular disruption is the critical factor in degeneration and, when a radial tear develops in the annulus, there is shrinkage with disorganization of the fibrous cartilage of the nucleus pulposus and replacement of the disk by dense fibrous tissue with cystic spaces (31,46–49). Annular tears, also properly called annular fissures, are separations between annular fibers, avulsion of fibers from their vertebral body insertions, or breaks through fibers that extend radially, transversely, or concentrically and involve one or many layers of the annular lamellae. The term tear or fissure describes the spectrum of such lesions and does not imply that the lesion is consequent to trauma (Fig 1). Although it has certainly been verified that annular disruption is a sequela of degeneration and certainly is often associated with it, its role as the causal agent of disk degeneration has certainly not been proved. MR is the most accurate anatomic method for assessing intervertebral disk disease. The signal intensity characteristics of the disk on T2-weighted images reflect changes caused by aging or degeneration. A classification scheme for lumbar intervertebral disk degeneration has been proposed that has reasonable intra- and interobserver agreement (50). To date, however, there has been no correlation between MR disk changes and patient's symptoms.
|
|
With loss of water and proteoglycans, the nucleus pulposus is desiccated and friable with yellow-brown discoloration. Its onion-skin appearance begins to unravel, and cracks, clefts, or crevices appear within the nucleus and extend into the annulus fibrosus. Fissuring, chondrocyte generation, and granulation tissue formation may be noted within the endplate, annulus fibrosis, and nucleus pulposus of degenerative disks, indicating attempts at healing (49) (Fig 2). Radiolucent collections (vacuum disk phenomena) representing gas, principally nitrogen, occur at sites of negative pressure produced by the abnormal spaces (51). The vacuum phenomenon within a degenerated disk is represented on SE images as areas of signal void (52). Whereas the presence of gas within the disk is usually suggestive of degenerative disease, spinal infection may (rarely) be accompanied by intradiscal or intraosseous gas (53).
|
As intervertebral osteochondrosis progresses, there may be calcification of the disk. Calcification has usually been described on MR images as a region of decreased or absent signal intensity. The loss of signal is attributed to a low mobile proton density, as well as, in the case of gradient-echo imaging, to its sensitivity to the heterogeneous magnetic susceptibility found in calcified tissue. There is, however, variability in signal intensity of calcium at various sequences, and the type and concentration of calcification are important factors. Hyperintense disks on T1-weighted MR images may be secondary to calcification (Fig 3) (54). For concentrations of calcium particulate up to 30% by weight, the signal intensity on standard T1-weighted images increased but then subsequently decreased (55,56). These data likely reflect particulate calcium reducing T1 relaxation times by a surface-relaxation mechanism. Hyperintensities that are affected by fat-suppression techniques have also been noted within intervertebral disks and are thought to be related to ossification with lipid marrow formation in severely degenerated or fused disks.
|
|
|
Degenerative Marrow Changes
The relationship among the vertebral body, endplate, annulus, and disk has been studied (57–59) by using both degenerated and chymopapain-treated disks as models. Signal intensity changes in vertebral body marrow (Fig 4) adjacent to the endplates of degenerated disks are a common observation on MR images and appear to take three main forms.
|
Type I changes demonstrate decreased signal intensity on T1-weighted images and increased signal intensity on T2-weighted images and have been identified in approximately 4% of patients scanned for lumbar disease (Fig 5), approximately 8% of patients after diskectomy (60,61), and in 40%–50% of chymopapain-treated disks, which may be viewed as a model of acute disk degeneration (58). Histopathologic sections of disks with type I changes show disruption and fissuring of the endplate and vascularized fibrous tissues within the adjacent marrow, prolonging T1 and T2. Enhancement of type I vertebral body marrow changes is seen with administration of gadopentetate dimeglumine that at times extends to involve the disk itself and is presumably related to the vascularized fibrous tissue within the adjacent marrow (Fig 6).
|
|
|
Type II changes are represented by increased signal intensity on T1-weighted images and isointense or slightly hyperintense signal on T2-weighted images and have been identified in approximately 16% of patients at MR imaging (Fig 7). Disks with type II changes also show evidence of endplate disruption, with yellow (lipid) marrow replacement in the adjacent vertebral body resulting in a shorter T1 (Fig 8).
|
|
|
Type III changes are represented by a decreased signal intensity on both T1- and T2-weighted images and correlate with extensive bony sclerosis on plain radiographs. The lack of signal in the type III change no doubt reflects the relative absence of marrow in areas of advanced sclerosis (Fig 9). Unlike type III, types I and II changes show no definite correlation with sclerosis at radiography (59). This is not surprising when one considers the histology; the sclerosis seen on plain radiographs is a reflection of dense woven bone within the vertebral body, whereas the MR changes are more a reflection of the intervening marrow elements.
|
|
Similar marrow changes have also been noted in the pedicles. While originally described as being associated with spondylolysis, they have also been noted in patients with degenerative facet disease and pedicle fractures. Again, the changes are probably a reflection of abnormal stresses, be they loading or motion (62,63).
Degenerative Facet and Ligamentous Changes
The superior articulating process of one vertebra is separated from the inferior articulating process of the vertebra above by a synovium-lined articulation, the zygoapophyseal joint. Like all diarthrodial synovium lined joints, the lumbar facet joints are predisposed to arthropathy with alterations of the articular cartilage. With disk degeneration and loss of disk space height, there are increased stresses on the facet joints with craniocaudal subluxation resulting in arthrosis and osteophytosis. The superior articular facet is usually more substantially involved. Facet arthrosis can result in narrowing of the central canal, lateral recesses, and foramina and is an important component of lumbar stenosis (Fig 10). However, it has been proposed that facet arthrosis may occur independently and be a source of symptoms on its own (64,65). Synovial villi may become entrapped within the joint with resulting joint effusions. The mechanism of pain may be related to nerve root compression from degenerative changes of the facets or by direct irritation of pain fibers from the innervated synovial linings and joint capsule (65). Osteophytosis and herniation of synovium through the facet joint capsule may result in synovial cysts, although the etiology of these facet joint cysts is unclear (Fig 11). There is a more straightforward relationship of synovial cysts with osteoarthritis and the instability of the facet joints than degeneration of the intervertebral disk alone. In a review of patients with degenerative facet disease, synovial cysts occurred at anterior or intraspinal location in 2.3% of cases and posterior or extraspinal location in 7.3% (66).
|
|
|
|
|
|
|
The important ligaments of the spine include the anterior longitudinal ligament, the posterior longitudinal ligament, the paired sets of ligamenta flava (connecting the laminae of adjacent vertebrae), intertransverse ligaments (extending between transverse processes), and the unpaired supraspinous ligament (along the tips of the spinous processes). As these ligaments normally provide stability, any alteration in the vertebral articulations can lead to ligamentous laxity with subsequent deterioration. Loss of elastic tissue, calcification and ossification, and bone proliferation at sites of ligamentous attachment to bone are recognized manifestation of such degeneration.
Excessive lordosis or extensive disk space loss in the lumbar spine leads to close approximation and contact of spinous processes and to degeneration of intervening ligaments (67,68). Histologically, granulomatous reaction and perivascular cellular infiltration characterize the condition (Fig 12).
|
Morphologic and Functional Sequellae
Common, potential complications of degenerative disk disease include alignment abnormalities, intervertebral disk displacement, and spinal stenosis. Various types of alignment abnormalities can exist alone or in combination, but the two most frequent are segmental instability and spondylolisthesis.
Instability
Segmental instability can result from degenerative changes involving the intervertebral disk, vertebral bodies, and facet joints that impair the usual pattern of spinal movement, producing motion that is irregular, excessive, or restricted. It can be translational or angular.
Spondylolisthesis results when one vertebral body becomes displaced relative to the next most inferior vertebral body. The most common types include degenerative, isthmic, iatrogenic, and traumatic. Degenerative spondylolisthesis is seen usually with an intact pars interarticularis, is related primarily to degenerative changes of the apophyseal joints, and is most common at the L4-5 vertebral level (Fig 10). Predilection for degenerative spondylolisthesis at that level is thought to be related to the more sagittal orientation of the facet joints, which makes them increasingly prone to anterior displacement. Degenerative disk disease may predispose to or exacerbate this condition secondary to narrowing of the disk space, which can produce subsequent malalignment of the articular processes and lead to rostrocaudal subluxation.
Herniation
Herniation refers to localized displacement of nucleus, cartilage, fragmented apophyseal bone, or fragmented annular tissue beyond the intervertebral disk space. The disk space is defined rostrally and caudally by the vertebral body endplates and peripherally by the outer edges of the vertebral ring apophyses, exclusive of osteophytic formations. The term localized contrasts with the term generalized, the latter being arbitrarily defined as greater than 50% (180°) of the periphery of the disk (28) (Fig 13).
|
|
Displacement, therefore, can occur only in association with disruption of the normal annulus or, as in the case of intravertebral herniation (Schmorl node) (Fig 14), a break in the vertebral body endplate. Since details of the integrity of the annulus are often unknown, the diagnosis of herniation is usually made by observation of displacement of disk material beyond the edges of the ring apophyses that is localized, meaning less than 50% (180°) of the circumference of the disk.
|
|
Localized displacement in the axial (horizontal) plane can be focal, signifying less than 25% of the disk circumference, or broad based, meaning between 25% and 50% of the disk circumference. Presence of disk tissue circumferentially (50%–100%) beyond the edges of the ring apophyses may be called bulging and is not considered a form of herniation.
A disk may have more than one herniation. The term herniated disk does not imply any knowledge of etiology, relation to symptoms, prognosis, or need for treatment. When data are sufficient to make the distinction, a herniated disk may be more specifically characterized as protruded or extruded. These distinctions are based on the shape of the displaced material. Protrusion is present if the greatest distance, in any plane, between the edges of the disk material beyond the disk space is less than the distance between the edges of the base in the same plane (Fig 15). Extrusion is present when, in at least one plane, any one distance between the edges of the disk material beyond the disk space is greater than the distance between the edges of the base in the same plane or when no continuity exists between the disk material beyond the disk space and that within the disk space (Fig 16). Extrusion may be further specified as sequestration if the displaced disk material has lost completely any continuity with the parent disk. The term migration may be used to signify displacement of disk material away from the site of extrusion, regardless of whether it is sequestrated or not (Fig 17).
|
|
|
|
|
Herniated disks in the craniocaudal (vertical) direction through a break in the vertebral body endplate are referred to as intravertebral herniations (Fig 14). Nonacute Schmorl-node intrabody herniations are common spinal abnormalities regarded as incidental observations. They have been reported in 38%–75% of the population (69,70). While intrabody herniations may occur secondary to endplate weakness related to bone displasia, neoplasms, infections, or any process that weakens the endplate or the underlying bone, most intrabody herniations probably form after axial loading trauma, with preferential extrusion of nuclear material through the vertebral endplate rather than an intact and normal annulus fibrosis. It has been suggested that asymptomatic intrabody herniations may be traceable to a specific occurrence of acute nonradiating low back pain in the patient's history, which supports the concept that intrabody herniations (Schmorl nodes) occur through sites of endplate fracture. Type I vertebral body marrow changes have been described surrounding the acute interbody herniations (71).
Stenosis
Spinal stenosis was defined in 1975 as any type of narrowing of the spinal canal, nerve root canals, or intervertebral foramina (72). Two broad groups have been defined: acquired (usually related to degenerative changes) and congenital or developmental. Developmental stenosis can be exacerbated by superimposed acquired degenerative changes. In the acquired type, there has been no association between the severity of pain and the degree of stenosis. The most common symptoms are sensory disturbances in the legs, low back pain, neurogenic claudication, weakness, and relief of pain by bending forward. The imaging changes are in general more extensive than expected from the clinical findings (73). Patients with symptoms referable to spinal stenosis tend to have narrower spines than asymptomatic patients. While there does appear to be a correlation between cross-sectional area and midsagittal measurements in patients with symptomatic spinal stenosis, absolute values and correlation between measurements and symptoms appear to be lacking. The degree of stenosis is not static, and extension worsens the degree of central and foraminal stenosis by 11%, while flexion appears to improve it by an average of 11%. Segmental instability, which can cause static and dynamic stenosis, is considered a cause of low back pain but is poorly defined (74). Some evidence suggests that disk degeneration, narrowing of the spinal canal, and degenerative changes in the facets and spinal ligaments contribute to stenosis and that instability increases with age. Unfortunately, there do not appear to be reliable prognostic imaging findings that would correlate with surgical success or even whether patients would benefit from surgery (75).
Technical Considerations for MR Imaging
Traditional clinical imaging has emphasized orthogonal T1- and T2-weighted imaging for morphologic assessment of the discovertebral complex. These sequences also provide an evaluation of the signal intensity changes associated with degenerative disk disease. Fast SE T2-weighted images have replaced conventional T2-weighted images because of their shorter acquisition times, but they provide no increased diagnostic advantage. Short inversion time inversion-recovery or fat-suppressed T2-weighted images have been added by many groups, ours included, because it is believed they are more sensitive to marrow and soft-tissue changes.
While these standard sequences remain the mainstay of diagnostic imaging of the spine, new techniques continue to be evaluated in hopes of providing stronger correlation between imaging findings and patient symptoms. The utility of many of these techniques for the routine evaluation of degenerative disk disease remains unknown, and the number of subjects in which they have been evaluated remains small. Nevertheless, these approaches may be important for redefining the direction of spinal imaging away from strictly anatomic one to one that combines more physiologic and functional information (76). Techniques that have been evaluated to greater or lesser degrees of success include assessment of spinal motion (dynamic imaging, kinetic assessment, or axial loading), diffusion imaging (water or contrast agents), MR neurography, spectroscopy, functional MR of the spinal cord, and ultrashort echo-time imaging. Of the variety of techniques available, only MR neurography and dynamic imaging have expanded beyond the experimental phase and have demonstrated specific clinical utility (albeit in niche or select populations).
Dynamic Imaging
The utility of dynamic spine MR remains unclear, in part due to the varied methods used. Methods to date include axial loading in the supine position by means of a harness that is attached to a nonmagnetic compression footplate with nylon straps that can be tightened or use of an upright open MR system that allows flexion and extension imaging (77,78). Dynamic MR has been used to evaluate the occurrence of occult herniations, which may not be visible or be less visible when the patient is supine, to measure motion between spinal segments, and to measure the canal or foraminal diameter when subjected to axial loading (76,78–81). Hiwatashi et al (80) evaluated 200 patients with clinical symptoms of spinal stenosis and found 20 patients with detectable differences in caliber of the dural sac on routine and axial loaded studies. In five of these selected patients, all three neurosurgeons involved in the clinical evaluation changed their treatment decisions from conservative to decompressive surgery. While a small subset of patients may benefit from this type of evaluation, the benefit appears small for the added machine time and patient discomfort.
Neurography
A large and varied literature exists concerning the use of MR neurography for the evaluation of peripheral nerves, including brachial and lumbar plexi. Thin-section MR neurography uses a high-resolution T1 imaging for anatomic detail and fat-suppressed T2-weighted or short inversion time inversion-recovery imaging to show abnormal nerve hyperintensity. Several reviews exist on this subject (82–84). The technique is capable of depicting a wide variety of pathologic conditions involving the sciatic nerve, such as compression, trauma, hypertrophy, neuroma, and tumor infiltration (85,86). MR neurography demonstrating piriformis syndrome (piriformis muscle asymmetry and sciatic nerve hyperintensity) has 93% specificity and 64% sensitivity (87).
Ultrashort Echo-time Imaging
Typical clinical MR imaging does not allow evaluation of tissues with very short relaxation times, since echo times are on the order of 8–15 msec. Ultrashort echo-time sequences have been preliminarily evaluated for a number of tissues, including the spine. These sequences have echo times as short as 0.08 msec. The images show normal contrast enhancement, with high signal intensity from longitudinal ligaments, endplate, and interspinous ligaments (88–90).
Diffusion
Several authors have evaluated the apparent diffusion coefficient (ADC) in normal and degenerated intervertebral disks. Antoniou et al (91) evaluated the ADC of cadaveric human disks related to matrix composition and matrix integrity by using a stimulated echo sequence. They found the ADCs in healthy subjects were significantly greater in the nucleus pulposus than in the annulus fibrosis. The ADCs were noted to generally decrease with degeneration grade and age in the nucleus. A similar correlation of ADC measurements and annular degeneration was not found. The most notable correlations were observed between the ADCs of nucleus pulposus and the water and glycoaminoglycan contents. Kealey et al (92) evaluated 39 patients with multishot SE echo-planar technique. They found a significant decrease in ADC of degenerated disks compared with that of normal disks. Kurunlahti et al (93) evaluated the ADC of disk and lumbar magnetic resonance angiograms in 37 asymptomatic volunteers. The lumbar artery status correlated with the diffusion values within the disks, suggesting that impaired blood flow may play an important role in disk degeneration. Kerttula et al (94) compared disk ADC values in normal controls with those in patients with prior compression fractures (at least 1 year previously) and found ADC values in x and y directions decreased in degenerated disks and in disks of normal signal intensity in the trauma area.
Diffusion-tensor imaging has been evaluated for imaging of the annulus fibrosus (95) and potentially for imaging defects or disruptions within the annulus (76). Differences in diffusion have been demonstrated for the intervertebral disk in compressed versus uncompressed states (96).
Intravenous contrast enhancement may also be used to assess diffusion into the intervertebral disk. Normal disks slowly enhance after contrast material injection, which may be as much as 36% in animal models. This enhancement is modified by the type of contrast agent (ionic vs nonionic) and molecular weight (97,98). Ionic material diffuses less rapidly into the disk than does nonionic media. Degenerated disks with decreased glycoaminoglycan have more intense and rapid enhancement (99). Disk enhancement has been documented in normal and degenerated human lumbar disks (100).
| SYMPTOMS |
|---|
| |
|---|
The etiology of symptoms in patients with degenerative disk disease is diverse, and there is often ambiguity in the diagnosis (101). The symptom complexes are more often characterized by variability and change rather than predictability and stability (102).
The most common symptom is pain. Anatomic areas of the spine can serve as sites of pain generation through intrinsic innervation or acquired innervation as a product of soft-tissue reparation. Mechanisms, which often act in combination, include (a) instability with associated disk degeneration, facet hypertrophy, or arthopathy; (b) mechanical compression of nerves by bone, ligament, or disk material; and (c) biochemical mediators of inflammation and/or pain.
It is important to reemphasize that disk degeneration per se is not painful, and in fact has a very high prevalence in the asymptomatic population. In addition, imaging findings of degenerative disk disease do not help predict a subsequent symptom development over time (103).
Mechanical compression or deformity of nerve roots as a cause of pain or nerve dysfunction is the classic concept related to displacement and effacement of neural tissue by disk herniation and dates to the observation of Mixter and Barr (104). Similar mechanical compression or traction mechanisms may be involved with instability or stenosis. A variety of morphologic changes occur in the nerve root with compression, including venous stasis, edema, and ultimately intraneural and perineural fibroses. Compression-induced impairment of both arterial and venous supply is one mechanism for nerve root dysfunction. Intraneural edema can occur even at low compression pressure levels (105). Mechanical compression itself may also be capable of producing changes in nerve impulses, which could be interpreted by the central nervous system as pain (106). However, the concept of neural compression by itself is inadequate to explain part or all of many symptom complexes.
The perplexing clinical scenario of patients who complain of incapacitating back pain, but may have no overt morphologic abnormality, has given rise to the concept of the disk as a pain generator. This was classically described by Crock (107) as "chronic internal disc disruption syndrome" (108,109). Many different names have been given to this idea, which becomes more confusing when combined with the various diagnostic tests that are used in an attempt to diagnose this protean syndrome. Additional terms in the literature include internal annular tear, internal disk disruption, black disk disease, and discogenic pain. In a normal human lumbar disk, nerve endings can be found only in the periphery of the annulus, and the pain fibers are part of the sympathetic chain via the sinuvertebral nerve (110–112). This innervates the outer layer of the annulus fibrosis. However, in very degenerated disks, nerves may even penetrate into the nucleus pulposus (113). Potentially, stimulation of these fibers can occur not only from direct disruption and mechanical pressure on the annulus but also from various breakdown products of the nucleus pulposus or secondarily upregulated inflammatory mediators. Discography has been cited as the reference standard for the diagnosis of discogenic pain, but what it means in terms of patient care has never been prospectively tested.
The concept of disk tissue producing an inflammatory response is not new, but has become more sophisticated and targeted with the application of monoclonal antibody technology, and other assay techniques (114) demonstrated chemical radiculitis, which was thought related to nuclear material and its glycoproteins, as being highly irritant to nerve tissue. McCarron et al (115), using a dog model, demonstrated in 1987 that autogenous placement of nucleus pulposus into the epidural space caused acute and chronic inflammatory reaction, with influx of histocytes and fibroblasts. Kayama et al (116) and Olmarker et al (117) demonstrated that nucleus pulposus applied to spinal nerves induces a wide variety of functional, vascular, and morphologic abnormalities, often followed by intraradicular fibrosis and neural atrophy. Nucleus pulposus can cause an inflammatory reaction with leukotaxis and increased vascular permeability (118). Direct placement of nuclear material is not necessary in animal models to induce an inflammatory response, but simply an incision of the annulus fibrosus can produce morphologic and functional changes in the adjacent nerves, such as increased capillaries and reduced nerve conduction velocities (116), with the presumed mechanism of disk material leakage into the epidural space. Monoclonal antibody staining of disk material has shown that the cells demonstrate an immunophenotype of inflammatory response, that is, macrophages (119). As a manifestation of this inflammatory response, higher systemic plasma levels of C-reactive protein have been found in patients with sciatica versus healthy controls (120).
Multiple studies have demonstrated vascularized granulation tissue surrounding the cartilage component of disk herniations (121,122), which correspond to the common enhanced MR findings of peripheral enhancement surrounding nonenhancing lumbar disk extrusions in unoperated patients. Blood vessels have been demonstrated in up to 91% of herniations, being most prevalent with disk sequestrations (123). Gronblad et al (124), using monoclonal antibodies, evaluated the types of inflammatory cells found with disk herniations and found them to be dominated by macrophages. There was also evidence of IL-1ß expression, an important proinflammatory cytokine.
Disk cells are also capable of expressing other proinflammatory substances, such as TNF- , which can produce radicular morphologic abnormalities similar to those seen with nucleus pulposus application (125). TNF-
, which can produce radicular morphologic abnormalities similar to those seen with nucleus pulposus application (125). TNF- is overexpressed in degenerated disks and is a proinflammatory cytokine affecting matrix metalloproteinases (MMP) expression and increasing prostaglandin E2. Weiler et al (126) demonstrated TNF-
is overexpressed in degenerated disks and is a proinflammatory cytokine affecting matrix metalloproteinases (MMP) expression and increasing prostaglandin E2. Weiler et al (126) demonstrated TNF- in cross-sections of human disks and found synthesis of TNF-
in cross-sections of human disks and found synthesis of TNF- in annular and disk regions, increased TNF-
in annular and disk regions, increased TNF- with symptomatic disk disease, and TNF-
with symptomatic disk disease, and TNF- expression associated with increasing disk degeneration. Olmarker and Rydevik (127) showed that inhibition of TNF-
expression associated with increasing disk degeneration. Olmarker and Rydevik (127) showed that inhibition of TNF- prevented thrombus formation and intraneural edema and reduced nerve conduction velocity. This set the stage for an open label trial of anti-TNF therapy in patients with sciatica (128–130). Infliximab (Remicade; Centocor, Malvern, Pa), a chimeric monoclonal human and mouse antibody, inhibits TNF-
prevented thrombus formation and intraneural edema and reduced nerve conduction velocity. This set the stage for an open label trial of anti-TNF therapy in patients with sciatica (128–130). Infliximab (Remicade; Centocor, Malvern, Pa), a chimeric monoclonal human and mouse antibody, inhibits TNF- –induced infiltration of leukocytes to the site of injury. A single infusion of infliximab produced a rapid beneficial effect on pain, which persisted over 1 year, at the 3 mg/kg dose level. Sustained improvement was also demonstrated with the subcutaneous injection of another anti-TNF agent, etanercept (Enbrel; Immunex-Amgen, Thousand Oaks, Calif) (128). While intriguing, the off-label use of these TNF inhibitors is not recommended, since little data are currently available and only a very small number of patients have been treated. This trial does point out the direction of research for treatment of disk disease, with specific targeting of inflammatory pathways.
–induced infiltration of leukocytes to the site of injury. A single infusion of infliximab produced a rapid beneficial effect on pain, which persisted over 1 year, at the 3 mg/kg dose level. Sustained improvement was also demonstrated with the subcutaneous injection of another anti-TNF agent, etanercept (Enbrel; Immunex-Amgen, Thousand Oaks, Calif) (128). While intriguing, the off-label use of these TNF inhibitors is not recommended, since little data are currently available and only a very small number of patients have been treated. This trial does point out the direction of research for treatment of disk disease, with specific targeting of inflammatory pathways.
A wide variety of inflammatory agents are capable of expression from both migratory macrophages into the site of herniation and directly from stimulated chondrocytes (131). Burke et al (132) found increased levels of IL-6, IL-8, prostaglandin E2, and monocyte chemoattractant protein-1 in disk extracts of patients undergoing fusion for discogenic pain. Monocyte chemoattractant protein-1 is a CC chemokine that contributes to the activation and recruitment of macrophages and is expressed by chrondrocytes that are stimulated by other cytokines and some MMPs. Disk tissue is biologically active and can respond to a proinflammatory stimulus by secreting IL-6, IL-8, and prostaglandin E2, but not TNF- . In a rabbit disk herniation model, however, Yoshida et al (133) demonstrated infiltrating macrophages at day 3 postoperatively, with intervertebral disk cells producing TNF-
. In a rabbit disk herniation model, however, Yoshida et al (133) demonstrated infiltrating macrophages at day 3 postoperatively, with intervertebral disk cells producing TNF- and IL-1ß on day 1 and monocyte chemoattractant protein-1 on day 3. In a mouse-derived co-culture system of disk material and macrophages, Kato et al (134) also demonstrated upregulation of TNF-
and IL-1ß on day 1 and monocyte chemoattractant protein-1 on day 3. In a mouse-derived co-culture system of disk material and macrophages, Kato et al (134) also demonstrated upregulation of TNF- messenger RNA and protein expression as the first point of the inflammatory cascade. The TNF-
messenger RNA and protein expression as the first point of the inflammatory cascade. The TNF- –dependent glycoprotein, TNF-
–dependent glycoprotein, TNF- –stimulated gene-6 (TSG-6), which is found in inflammatory diseases of related connective tissues, has been demonstrated in 98% of disk herniations in one series (135).
–stimulated gene-6 (TSG-6), which is found in inflammatory diseases of related connective tissues, has been demonstrated in 98% of disk herniations in one series (135).
Another component of the inflammatory response involves the matrix-degrading enzymes called MMPs. There are approximately 25 MMPs in five classes based on the specificity of their substrate. These enzymes degrade the extracellular matrix at physiologic pH levels. They are released by resident cells such as fibroblasts, macrophages in herniated disks, and chondrocytes from protrusions and nonherniated disk (136). MMPs can play a direct role in disk degeneration by causing matrix proteolysis and disk resorption and have an indirect role in angiogenesis. MMPs involved in disk degeneration include MMP-1 (collagenase); MMP-3 (stromelysin-1); MMP-9 (gelatinase B); and MMPs-2, -7, -8, and -13 (137). Cells within granulation tissue in disk herniations express MMP-1 and MMP-3 (138,139). MMP-3, but not MMP-7 (matrilysin), appears necessary for disk resorption, although the mechanism may be indirect and correlates with macrophage infiltration (140). The angiogenic properties are more indirect, with endothelial cell migration occurring only after a proteolytic reaction produced by MMP-3 (141). Cytokines leading to MMP production within the disk herniation may then result in angiogenesis and disk resorption. Given the wide variety of MMPs present, it is likely that there is a cascade of interacting proteases for different components of the disk matrix involved in disk resorption and degeneration.
Many other molecules have also shown to be present in degenerated or herniated disks that may play additional roles in the inflammatory cascade, such as intercellular adhesion molecule-1, fibroblast growth factor, and vascular endothelial growth factor (142,143). These two latter agents contribute to neoangiogenesis. Vascular endothelial growth factor appears to require TNF- for induction. Additionally, it is a potent inducer of plasmin and results in activation of a variety of MMPs. Interaction between the vascular endothelial growth factor and MMPs could promote disk matrix degeneration, as well as neovascularization of herniations.
for induction. Additionally, it is a potent inducer of plasmin and results in activation of a variety of MMPs. Interaction between the vascular endothelial growth factor and MMPs could promote disk matrix degeneration, as well as neovascularization of herniations.
Nerve fibers have been identified in the outer third of the annulus in the normal state, but may extend into the inner annulus and nucleus pulposus, accompanied by blood vessels, in chronic back pain patients (113). These nerves also stain for substance P, a putative nociceptive neurotransmitter, along with calcitonin gene-related peptide and vasoactive intestinal peptide. These small nonmyelinated nerve fibers grow into the disk in areas with local production of nerve growth factor, which is produced by the neoangiogenesis of the disk material (113). Coupled with nerve ingrowth and angiogenesis is the production of inflammation by liberation of the potent inflammatory agent phospholipase A2, which catalyzes the hydrolysis of phosphoglyceride, an important membrane constituent (144,145). This production of phospholipase A2 is induced by, among other signals, the presence of IL-1 and TNF- , with the subsequent upregulation of the arachadonic acid cascade, which produces prostaglandin E2 and leukotrienes (146,147). Prostaglandin E2 production has the critical rate-limiting enzyme cyclooxygenase-2, which appears to be primarily upregulated during inflammation (148). The other branch of the arachadonic acid cascade is the production of leukotrienes by means of the enzyme lipoxygenase. A bell-shaped pain behavior dose response curve has been demonstrated for intraneural injection of TNF-
, with the subsequent upregulation of the arachadonic acid cascade, which produces prostaglandin E2 and leukotrienes (146,147). Prostaglandin E2 production has the critical rate-limiting enzyme cyclooxygenase-2, which appears to be primarily upregulated during inflammation (148). The other branch of the arachadonic acid cascade is the production of leukotrienes by means of the enzyme lipoxygenase. A bell-shaped pain behavior dose response curve has been demonstrated for intraneural injection of TNF- and IL-1ß in a rat model, peaking at doses equivalent to those of endogenous cytokines released locally after nerve injury. An increase in perineural macrophages was also observed, particularly for IL-1ß (149).
and IL-1ß in a rat model, peaking at doses equivalent to those of endogenous cytokines released locally after nerve injury. An increase in perineural macrophages was also observed, particularly for IL-1ß (149).
Sensory and motor deficits would appear to be the result of both a combination of mechanical deformation and the presence of inflammation. A variety of mechanisms and mediators are involved in the inflammatory side of the equation, and a brief general overview is attempted in Figure 18. Clearly, the etiology of pain in degenerative disease is much more complex than a simple mechanical explanation, and work on these other factors will hopefully bring us a greater understanding of the relationship between morphologic alteration and clinical symptoms.
|
| IMPORTANCE OF IMAGING FINDINGS |
|---|
| |
|---|
The role of an imaging test is to provide accurate morphologic information and influence therapeutic decision making (150). A necessary component, which connects these two purposes, is accurate natural history data.
Modern imaging has made important strides in supporting the first goal, accurate morphologic information. Not only are morphologic changes depicted in ever-increasing anatomic detail, but additional information from the imaging study has been made available that is helping us to understand cellular and biochemical alterations. The ability to better characterize these alterations should provide a means of more accurately stratifying patient changes that may allow a more accurate understanding of etiology.
Any study looking at the natural history of degenerative disk disease, prognostic value of imaging, or its effect on therapeutic decision making will be confounded by the high prevalence of morphologic change in the asymptomatic population (151–153). A 20%–28% of asymptomatic patients demonstrate disk herniations, and the majority have evidence of additional degenerative disk disease (151–153). These findings are not only nonpredictive at the moment, but prospectively as well. In a 7-year follow-up of the Borenstein et al (103) original patient group, the original MR findings were not predictive of the development or duration of low back pain.
As to natural history, some information is available. Degenerative disk space narrowing, facet disease, and stenosis tend to slowly progress over time. Eventual stabilization of the three-joint discovertebral complex is thought to be part of the natural history of degenerative disease, and it is assumed to be accompanied by a decrease in pain. These impressions, however, are anecdotal and have not been tested by a formal natural history study. Some findings, such as disk herniation and degenerative marrow changes, are known to change. Multiple studies in which computed tomography or MR imaging has been used have shown that the size of disk herniations, especially larger ones, can reduce dramatically in patients undergoing conservative treatment (154,155).
In a study of symptomatic patients, the prevalence of disk herniation in patients with low back pain and those with radiculopathy at presentation was similar (156). There was a higher prevalence of herniation, 57% in patients with low back pain and 65% in patients with radiculopathy, than the 20%–28% prevalence reported in asymptomatic series (152,153). Disks characterized as extruded showed more marked regression in patients with both low back pain and radiculopathy. In general, one-third of patients with disk herniation at presentation had significant resolution or disappearance by 6 weeks and two-thirds by 6 months (155,156). The type, size, and location of herniation at presentation and changes in herniation size and type over time did not correlate with outcome. In fact, the presence of a herniation at a MR was a positive prognostic finding (156).
Interestingly, not only do disk herniations have a tendency to regress, but also new or larger ones may appear after the onset of symptoms. In this study, 13% of patients in this symptomatic series developed new or larger disk herniations over a 6-week period. In looking at patients with low back pain or radiculopathy, MR did not have additive value over clinical assessment. No prognostic sign that might alter treatment versus clinical assessment alone was identified. The size and type of disk herniation and location and presence of nerve root compression, significant in terms of morphologic alteration, were not related to patient outcome. Likewise, the presence or absence of stenosis, facet disease, or degenerative marrow changes did not correlate with patient outcome (156).
This lack of prognostic value also appears to apply to the conservative management of spinal stenosis. There do not appear to be reliable prognostic imaging findings that would correlate with surgical success or even whether patients would benefit from surgery and spinal stenosis (75,157). A study of the qualitative morphologic features of the spinal canal dimensions and herniated disks has not proved helpful in predicting outcomes in patients with back pain and sciatica. Demographic and clinical features appear to predict outcome of nonsurgical treatment, whereas morphometric features of disk herniation and spinal canal are more powerful predictors of surgical outcome (158).
Degenerative marrow changes may also change over time. In all three types, there is always evidence of associated degenerative disk disease at the level of involvement. Type I changes may revert to normal or convert to type II changes, with time suggesting some stabilization of the degenerative process. Type II changes tend to be more stable but may convert to type I or a mixed combination of types I and II. When changes do occur in type II marrow, they are usually associated with evidence of additional or accelerated degeneration or a superimposed process such as infection or trauma.
The clinical importance of marrow changes associated with degenerative disk disease remains unclear. Type I changes seem to be associated with a higher prevalence of active low back pain symptoms. The exact etiologic mechanism or mechanisms, while unknown, have been thought related to some type of unusual stresses, micro- or macroinstabilility or microtrauma. Some studies of discography in patients with degenerative marrow changes have suggested that type I marrow changes are invariably associated with painful disks (159–162). Surgical studies have suggested that patients with type I marrow changes who undergo fusion for low back pain do better than those without endplate changes or type II patterns (159). The hypothesis is that type I degenerative marrow changes are related to or are indicators of some degree of instability. Authors of a surgical study looking at the prognostic value of type I marrow changes related to surgical outcome demonstrated that persistence of type I marrow changes after fusion was associated with significantly worse outcome (163). The authors speculate that type I changes may not only be an important criterion for surgery, but type I change disappearance may be an indicator of successful fusion and stabilization. Additional evidence to support that these changes are a reflection of a more active process related to microtrauma and instability is a surgical study that found that in the overwhelming majority of patients with type I marrow changes who undergo fixation and fusion, the marrow changes will convert to a normal marrow signal intensity or type II changes, with good clinical results (159).
| SURGERY |
|---|
| |
|---|
Degeneration of the intervertebral disk complex is a process that begins early in life and is a consequence of a variety of genetic, physiologic, and environmental factors, as well as normal aging. Given the ubiquitous nature of the process and its high prevalence in both symptomatic and asymptomatic individuals, the jump from identifying an anatomic derangement to proposing a symptom complex must be made with caution (2). There is an opportunity for imaging to further our understanding of the process.
What separates individuals with dramatic morphologic findings who have no symptoms from individuals with identical alterations who do? Understanding the relationship of etiologic factors, the morphologic alterations, which can be characterized at imaging, and the mechanisms of pain production and their interactions in the production of symptoms will require more accurate and reproducible stratification of patient cohorts. This may be a strong suit of imaging, the phenotyping of morphologic alterations to compare with the emerging genotyping work relative to etiology and clinical manifestations. The ultimate translational goal is the integration of this newfound understanding into the therapeutic decision-making process.
| ESSENTIALS |
|---|
| |
|---|
- Mechanical, traumatic, nutritional, and genetic factors all play a role in the cascade of disk degeneration.
- Reliable and reproducible terminology is critical to meaningful description of morphologic abnormalities.
- The etiology of pain in degenerative disease is more complex than a simple mechanical explanation.
- The prognostic value of imaging is confounded by the high prevalence of morphologic changes in the asymptomatic population.
- In patients with uncomplicated low back pain or radiculopathy, MR imaging may not have an additive value over clinical assessment.



































